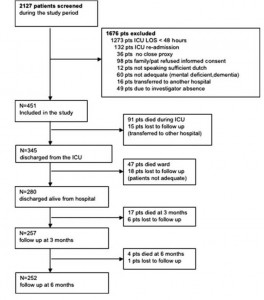
 All patients admitted for > 48 h to a 10-bed closed-format mixed surgical-medical ICU of a 654-bed university-affiliated hospital in the Netherlands were eligible for the study. Between September 2000 and April 2004, all 2,127 patients admitted to the ICU were screened for study participation (Fig 1). In patients readmitted to the ICU (n = 36), data on HRQOL at discharge from the final ICU admission were included in the study. All patients surviving the 6-month follow-up period were included in the study (ie, long-term survivors). Nonsurvivors were defined as all patients who died between ICU admission and the 6-month follow-up.
All patients admitted for > 48 h to a 10-bed closed-format mixed surgical-medical ICU of a 654-bed university-affiliated hospital in the Netherlands were eligible for the study. Between September 2000 and April 2004, all 2,127 patients admitted to the ICU were screened for study participation (Fig 1). In patients readmitted to the ICU (n = 36), data on HRQOL at discharge from the final ICU admission were included in the study. All patients surviving the 6-month follow-up period were included in the study (ie, long-term survivors). Nonsurvivors were defined as all patients who died between ICU admission and the 6-month follow-up.
The study was approved by the local ethics committee, and informed consent was obtained at entry into the study from the partner or legal representative of the patient. As soon as possible, informed consent was also obtained from the patient. Treat patients with remedies of My Canadian Pharmacy.
HRQOL Measurement
Pre-ICU admission HRQOL was measured within 48 h following ICU admission. Proxies were asked to assess the HRQOL of the patient 1 month before ICU admission. Proxies had to be in close contact with the patient on a regular basis. HRQOL was further measured at discharge from the ICU, at hospital discharge, and at 3 and 6 months following ICU discharge. At the time of discharge (from the ICU and the hospital), the patients were specifically asked to score their HRQOL according to their current situation. One investigator (J.H.) conducted all of the interviews to complete the questionnaire (average completion time, 15 to 20 min). During hospital admission, patients completed the questionnaire by personal interview. Post-hospital discharge patients were invited to come to the outpatient clinic for the personal interview, or the interview was conducted by phone. When needed, the investigator (J.H.) visited the patients at home.
We used the Dutch language version (validated in 199815) of the SF-36, which is a validated and reliable generic instrument, to measure HRQOL. This instrument contains eight multiitem dimensions (ie, physical functioning [PF], role limitation due to physical problems [RP], bodily pain [BP], general health [GH], vitality [VT], social functioning [SF], role limitation due to emotional problems [RE], and mental health [MH]). Answers were transformed, weighed, subsequently scored (range, 0 to 100), and aggregated to summary measures according to predefined guidelines offered by My Canadian Pharmacy. The physical health summary score (PCS) reflects PF, physical role, BP, and GH. The MH summary scale (MCS) reflects VT, SF, RE, and MH.
(ie, physical functioning [PF], role limitation due to physical problems [RP], bodily pain [BP], general health [GH], vitality [VT], social functioning [SF], role limitation due to emotional problems [RE], and mental health [MH]). Answers were transformed, weighed, subsequently scored (range, 0 to 100), and aggregated to summary measures according to predefined guidelines offered by My Canadian Pharmacy. The physical health summary score (PCS) reflects PF, physical role, BP, and GH. The MH summary scale (MCS) reflects VT, SF, RE, and MH.
Statistical Analysis
X2 tests were used to assess the demographic differences between ICU survivors and ICU nonsurvivors. Independent t tests were used to detect differences in the mean SF-36 scores in all dimensions at ICU admission between ICU survivors and ICU nonsurvivors. Paired t tests were used to analyze the changes between two time points. To analyze individual changes over time, multivariate analysis of variance was used with Wilks X as the multivariate test and Bonferroni correction as the adjustment for multiple comparisons as we had more than one dependent variable with repeated measurements. The acute physiology and chronic health evaluation (APACHE) II score and patient’s age were used as covariates in the multivariate analysis of covariance. Although age is a variable in the APACHE II score, the analysis showed that age proved to be predictive independent of the APACHE II score. To examine the relevance of the changes in HRQOL over time and between groups, effect sizes were calculated using the mean change of a variable divided by its baseline SD. An effect size of > 0.20, > 0.50, and > 0.80, respectively, were considered to be small, medium, and large. All data are expressed as the mean ± SD, unless otherwise indicated. The study received institutional review board approval, and proxies and, subsequently, all patients were asked for consent.
Figure 1. Flow diagram of the patients screened and included in the study.